CD33 Single Nucleotide Polymorphism Analysis for Gemtuzumab Ozogamicin (GO/Mylotarg™) Treatment Response for AML
- Mylotarg™ has been approved by the FDA for treatment of AML
- A single nucleotide polymorphism can predict response to Mylotarg™
- HematoLogics has developed a molecular diagnostic test to identify patients who will benefit from treatment with Mylotarg™
- CD33 SNP sequencing analysis will determine if the patient has a T-allele (non-responsive to Mylotarg™) or a CC-genotype (responsive to Mylotarg™)
Sample Case
Homozygous C>C allele allows the use of Gemtuzumab Ozogamicin (GO; Mylotarg) as an option for treatment of AML, while heterozygous C>T or homozygous T>T patients will not respond due to lack of CD33 binding site.
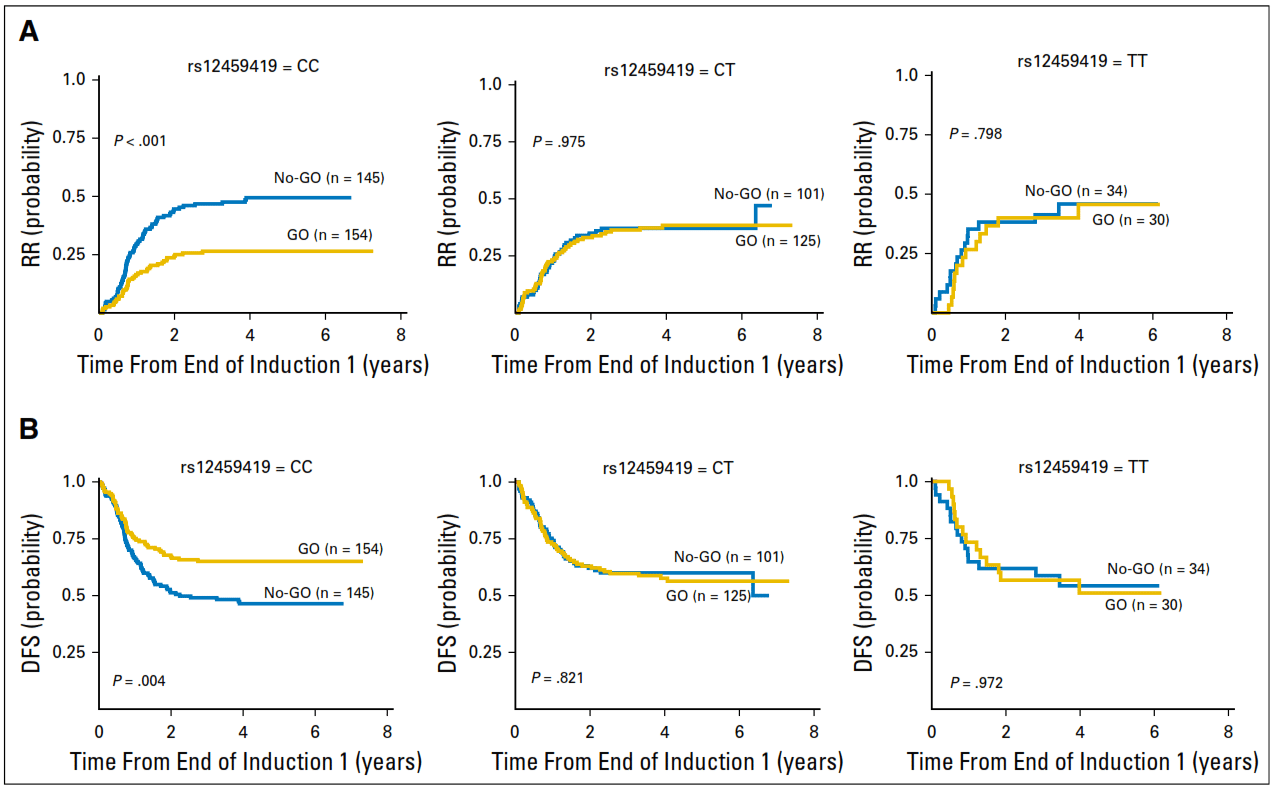
Lamba, J. K., Chauhan, L., Shin, M., Loken, M. R., Pollard, J. A., Wang, Y. C., S. Meshinchi, S. (2017). CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo acute myeloid leukemia: Report from randomized phase III children’s oncology group trial AAML0531. Journal of Clinical Oncology, 35(23), 674-2682. DOI: 10.1200/JCO.2016.71.2513
Published Studies:
Blood Journal Published February 29, 2012
- Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML
Journal of Clinical Oncology Published June 23, 2016
- CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo AML: report from randomized phase III Children’s Oncology Group Trial AAML0531
Leukemia Published February 23, 2009
- Coding Polymorphisms in CD33 and response to gemtuzumab ozogamicin in pediatric patients with AML
Lancet Oncology Published June 29, 2009
- Potential biomarker for acute myeloid leukemia therapy

